Are your metrology systems 21 CFR Part 11 compliant?
Estimated reading time 3 minutes
The importance of data integrity in the medical device industry
According to the US Food and Drug Administration (FDA), 7,252 medical products were recalled in 2020 — 41 per cent of these products were medical devices. Recalls are costly, both financially and to a business’ reputation, but also can pose a risk to patient safety, something all medical device businesses want to avoid. Here Jason McGlynn, commercial manager for Ireland at industrial metrology specialist The Sempre Group, explains how quality engineers can maintain data integrity — and stay 21 CFR Part 11 compliant — to prevent recalls.
Traceability is vital when manufacturing medical devices. Manufacturers must be able to provide information about every step of the process — including when each operation was completed, why, how and by who — to ensure product quality and safety.
What is 21 CFR Part 11?
Audit trails chronologically catalogue every process to provide records of operational activity during manufacturing. Auditors of devices that will be sold in the US market will check this trail to ensure that the process is fully traceable and there are no gaps where human error could cause issues, in accordance with 21 CFR Part 11.
This FDA regulation refers to how medical device companies use electronic documentation and signatures in their quality management systems. 21 CFR Part 11 was first published in 1997 to help companies in the medical device industry know how to use computers and software, maintain data safely and securely to ensure it is not corrupted or lost and trace changes to data to prevent and/or detect falsified records. It has been updated since, to reflect how technology use has changed in medical device manufacturing.
Full traceability
Not all 21 CFR Part 11 compliant equipment and software will provide a fully traceable audit trail. For example, a validated measurement system can take hundreds of measurements of a device and generate large reports of raw data so manufacturers can monitor quality. However, if quality engineers export that data and import it into another data analysis system, it leaves a gap. If there are no clear security controls when moving data manually between systems, there are multiple opportunities for human error to introduce faults that cannot be traced.
Moving data between systems can compromise data integrity — which the FDA defines as the completeness, consistency and accuracy of data and how it is recorded. Using an electronic quality management system (EQMS) can both ensure data integrity and reduce the risk of issues occurring. Information is automatically time stamped, alterations are recorded and information is stored in one central location.
Compliant equipment
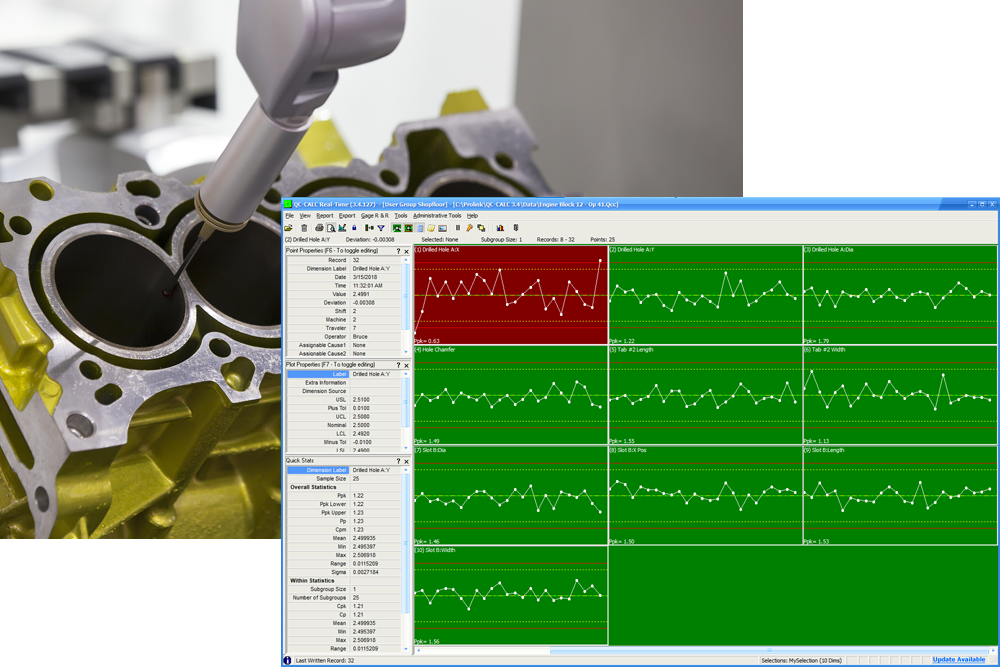
When choosing data collection software, opting for one that is 21 CFR Part 11 compliant will help ensure security and authenticity. Automated, real-time data collection software, such as the Prolink SPC Data Collection Software, otherwise known as QC-CALC, can extract real-time data from any device to enable full analysis and reporting. An easy-to-use system can provide total visibility and control of data across the entire manufacturing process. Another benefit is the ability to automatically generate password protected reports, which makes it easy to spot any changes during production, identify where they happened and why and take steps to investigate and fix the issue.
Effective quality management and reporting processes can help prevent expensive recalls that can damage a manufacturer’s reputation. A compliant electronic system also means that, if a recall does occur, medical device companies have the ability to refer to transparent and authentic data so that they can correct the issue quickly and effectively.
For advice on the best EQMS and metrology equipment for your medical device company, visit www.thesempregroup.com or call +44 (0)1452 915 072.


